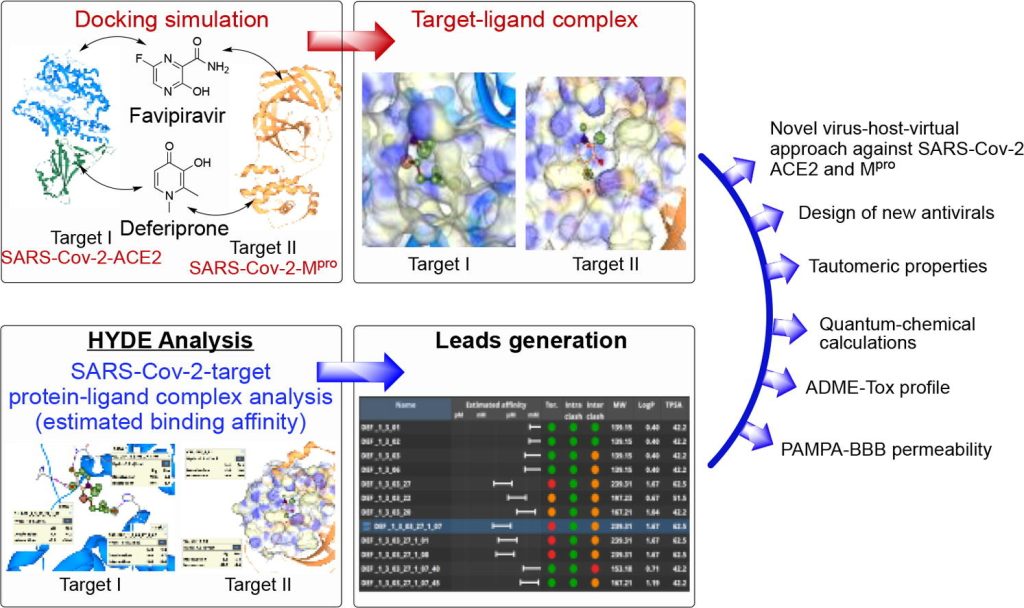
Coronavirus disease 2019 (COVID-19) still remains the most disastrous infection continuously affecting millions of people worldwide. Herein, we performed a comparative study between the anti-influenza drug favipiravir (FAV) and the anti-thalassemia drug deferiprone (DFP) in order to examine their potential as basic scaffolds for the generation of most effective and structurally novel antivirals. To conduct the initial molecular modelling and virtual screening steps, our recently proposed single crystal X-ray diffraction (SCXRD)/HYdrogen DEssolvation (HYDE) technology platform has been used. This platform allows molecular design, interactive prioritization and virtual evaluation of newly designed molecules, simultaneously affecting two COVID-related targets, including angiotensin-converting enzyme 2 (ACE2) as a host-cellular receptor (host-based approach) and the main protease (Mpro) enzyme of the spike glycoprotein of SARS-Cov-2 (virus-based approach). Based on the molecular docking results, DFP has shown higher binding affinity (Ki HYDE values) over FAV towards both biological targets. The tautomeric, physicochemical, and biological properties of FAV and DFP have been studied both experimentally and theoretically using molecular spectroscopy (UV-VIS absorption), parallel artificial membrane permeability assay, and cell biology (PAMPA and MTT assay), as well as DFT quantum chemical calculations. According to the obtained results, the enol tautomers of both compounds are considerably more stable in different organic solvents. However, the keto tautomer of FAV was estimated to be most preferable under physiological conditions, which is in good agreement with the molecular docking studies. The isolated crystal structure of DFP is in an excellent agreement with the computation in respect of the most stable tautomer. Combined single X-ray/molecular modeling studies including HYDE analyses provided not only insights into the protein-ligand interactions within the binding site of SARS-Cov-2-ACE2 and SARS-Cov-2-Mpro, but also a valuable information regarding the most stable enol tautomeric form of DFP that contributes to its estimated higher potency against these targets.