Herein, we demonstrate a working prototype of a conjugated proton crane, a reversible tautomeric switching molecule in which truly intramolecular long-range proton transfer occurs in solution at room temperature. The system consists of a benzothiazole rotor attached to a 7-hydroxy quinoline stator. According to the experimental and theoretical results, the OH proton is delivered under […]
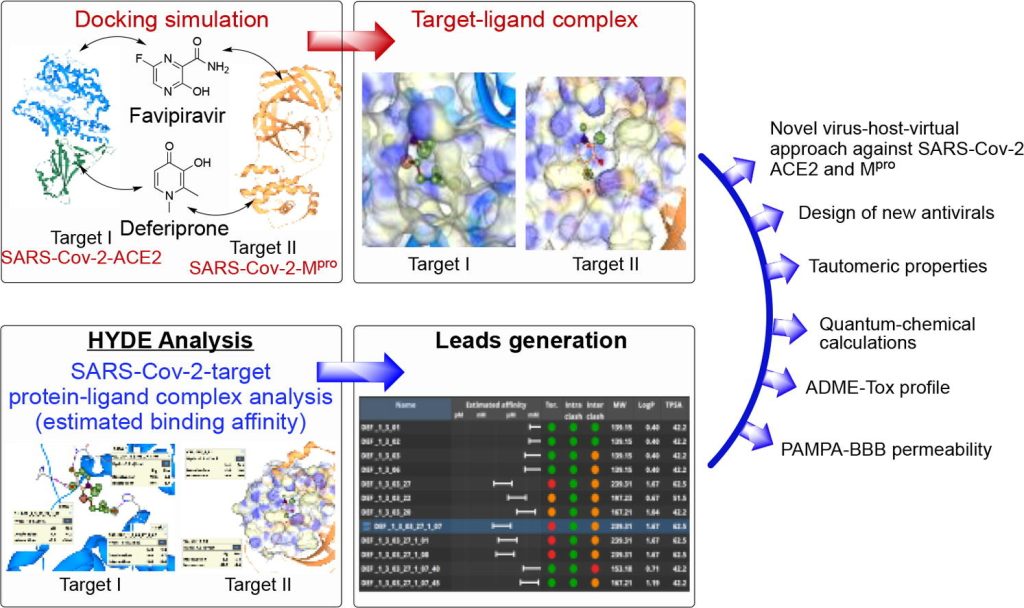
Coronavirus disease 2019 (COVID-19) still remains the most disastrous infection continuously affecting millions of people worldwide. Herein, we performed a comparative study between the anti-influenza drug favipiravir (FAV) and the anti-thalassemia drug deferiprone (DFP) in order to examine their potential as basic scaffolds for the generation of most effective and structurally novel antivirals. To conduct […]
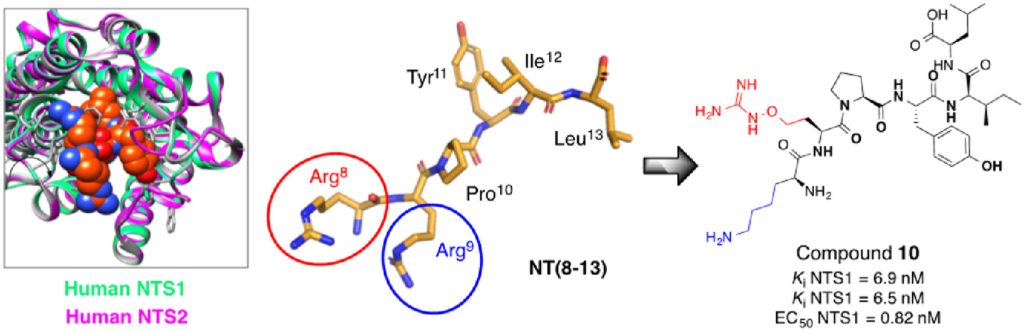
The modulatory interactions between neurotensin (NT) and the dopaminergic neurotransmitter system in the brain suggest that NT may be associated with the progression of Parkinson’s disease (PD). NT exerts its neurophysiological effects by interactions with the human NT receptors type 1 (hNTS1) and 2 (hNTS2). Therefore, both receptor subtypes are promising targets for the development […]
The tautomeric properties of favipiravir, a promising anti-COVID-19 drug, were investigated experimentally for the first time by using molecular spectroscopy (UV–Vis absorption, fluorescence and NMR), as well as DFT quantum–chemical calculations. According to the obtained results, the enol tautomer is substantially more stable in most of the organic solvents. In the presence of water, a […]
In the tautomeric Schiff bases, derived from 7-hydroxyquinoline, two competitive channels are possible upon excitation of the enol tautomer, namely proton transfer (PT) through intramolecular hydrogen bonding to the corresponding keto form and trans–cis isomerization around the azomethine double bond. The former leads to switching, based on twist-assisted excited state intramolecular PT, where the long-range […]
The twelfth edition of the Sofia Science Festival was held on October 8 and 9, 2022 in Sofia Tech Park. The Festival brings affordable and fun events that reveal how the wonders of science and the latest research from around the world enrich our lives, explain the familiar phenomena of our everyday lives, and bring […]
The ability of long-range proton transport by substitution of 7-hydroxyquinoline at the eighth position with sulfonamide and sulfonylhydrazone rotor units to act as a crane-arm has been studied. Different proton transport pathways triggered by different stimuli have been established depending on the structure of the crane-arms. Solvent-driven proton switching from OH to the quinoline nitrogen […]
The ground state tautomerism and excited state intramolecular proton transfer in two new molecular switches, namely N-(benzo[d]thiazol-2-yl)picolinamide and N-(benzo[d]thiazol-2-yl)isonicotinamide, are studied in acetonitrile by the combined use of steady state and time dependent spectroscopy and DFT calculations. Although, according to the theoretical calculations, two potential switching pathways are possible in N-(benzo[d]thiazol-2-yl)picolinamide, either through the pyridine […]
The acid dissociation constant of three benzimidazoles, namely 2,2′-bibenzo[d]imidazole, 2,5′-bibenzo[d]imidazole, and 5,5′-bibenzo[d]imidazole, have been investigated by means of density functional theory calculations in gas phase and in aqueous solution. The theoretical approach was validated by the comparing of predicted and experimentally determined pKa values in imidazole, benzimidazole, and 2-phenylbenzimidazole. From the studied compounds, 2,2′-bibenzo[d]imidazole was […]
We are very proud to announce that Dr Silvia Hristova, who defended her PhD thesis in our group in 2020, was awarded by The Eureka Foundation as young scientist with substantial contribution in the science. The thesis of Silvia considers the possibility of using controlled proton transfer as an elementary process in the molecular machinery. […]
- 1
- 2